1. Atomic Structure
The atomic structure is like the blueprint of an atom, which is the smallest unit of matter that makes up everything around us.
- What is an Atom?
An atom is made up of three main particles:- Protons: Positively charged particles located in the nucleus (the center of the atom).
- Neutrons: Neutral particles (no charge) also found in the nucleus.
- Electrons: Negatively charged particles that orbit around the nucleus in shells or energy levels.
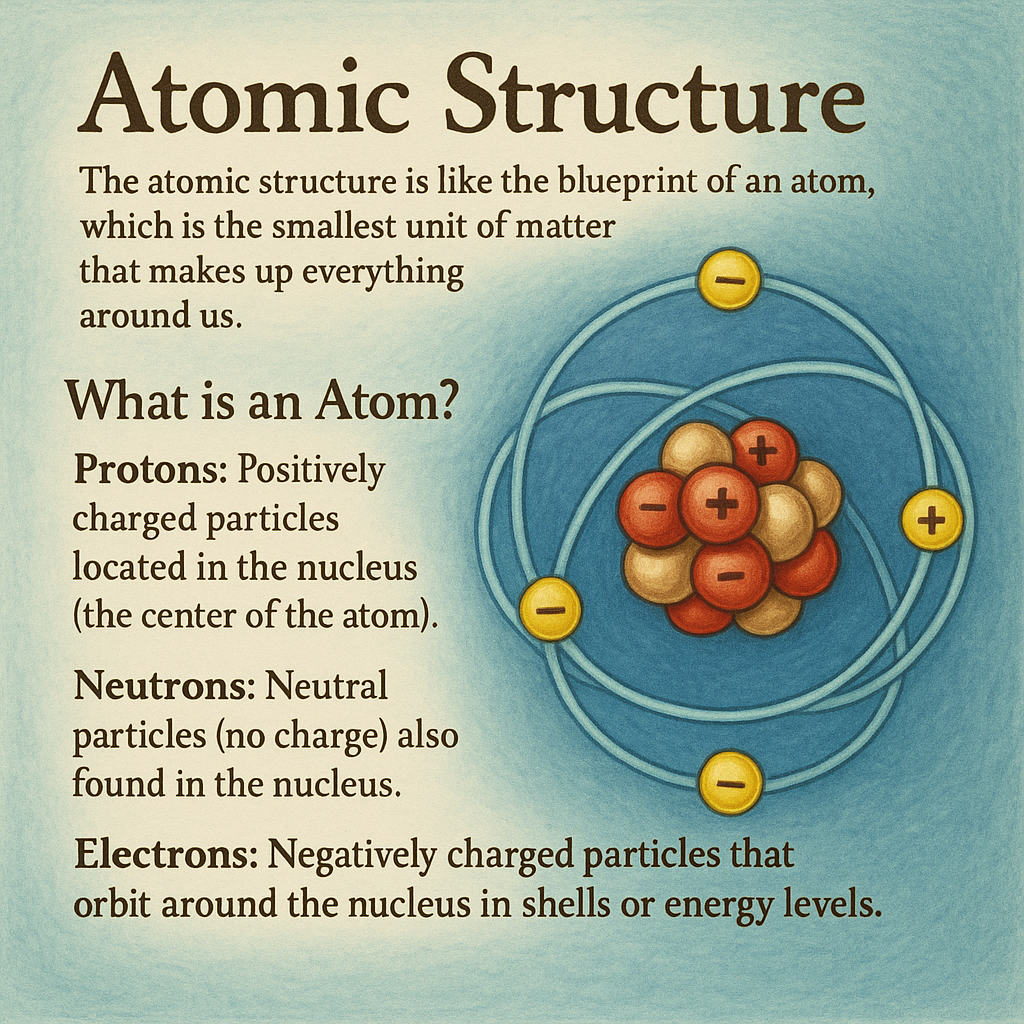
How is an Atom Structured?
- The nucleus (center of the atom) contains protons and neutrons.
- The electrons move around the nucleus in orbits (like planets orbiting the sun). These orbits are divided into energy levels (shells).
- The number of protons in the nucleus determines what element the atom is. For example:
- Hydrogen has 1 proton,
- Oxygen has 8 protons.
Atomic Number and Mass Number
- The atomic number is the number of protons in an atom, which also equals the number of electrons in a neutral atom.
- The mass number is the total number of protons and neutrons in an atom.
Example:
- In a Carbon (C) atom:
- Atomic number = 6 (because it has 6 protons)
- Mass number = 12 (because it has 6 protons and 6 neutrons)
2. Organic Chemistry
Organic chemistry is the branch of chemistry that studies carbon-based compounds. Almost all living things are made up of organic compounds, so organic chemistry is very important for life.
- What Makes a Compound Organic?
A compound is organic if it contains carbon atoms. In organic compounds, carbon atoms bond with other elements, such as hydrogen, oxygen, nitrogen, etc.
Key Features of Organic Compounds:
- Carbon atoms can form strong bonds with other carbon atoms, which allows for the creation of large and complex molecules (like those in living organisms).
- Organic compounds can be simple (like methane – CH₄) or complex (like DNA – a huge molecule with carbon atoms).
Common Organic Compounds:
- Hydrocarbons: Compounds made only of carbon and hydrogen. They can be:
- Alkanes: Simple hydrocarbons with single bonds, e.g., methane (CH₄).
- Alkenes and Alkynes: Hydrocarbons with double or triple bonds, e.g., ethene (C₂H₄) or ethyne (C₂H₂).
- Functional Groups: These are specific groups of atoms that give compounds their chemical properties. For example:
- Alcohols (contains –OH group, like ethanol (C₂H₅OH)).
- Carboxylic Acids (contains –COOH group, like acetic acid (CH₃COOH)).
Why is Organic Chemistry Important?
- Life: All living organisms are made from organic compounds (like proteins, fats, and sugars).
- Everyday Products: Things like plastics, medicines, food, and fuels are made from organic chemistry.
3. Chemical Reactions
A chemical reaction happens when substances change into new substances. This happens because the atoms of the original substances rearrange to form new substances with different properties.
What Happens During a Chemical Reaction?
- Bonds Break: The bonds between atoms in the reactants (starting substances) are broken.
- New Bonds Form: New bonds are made between the atoms to create the products (new substances).
Basic Types of Chemical Reactions:
- Combination Reaction: Two or more substances combine to form one new substance.
- Example:
(Hydrogen + Oxygen = Water)
- Example:
- Decomposition Reaction: A single substance breaks down into two or more simpler substances.
- Example:
(Hydrogen Peroxide breaks down into Water and Oxygen)
- Example:
- Displacement Reaction: One element displaces another in a compound.
- Example:
(Zinc displaces copper from copper sulfate)
- Example:
- Combustion Reaction: A substance reacts with oxygen to produce heat and light.
- Example:
(Methane + Oxygen = Carbon dioxide + Water + Heat)
- Example:
Balancing Chemical Equations:
In a chemical reaction, the number of atoms of each element must be the same on both sides of the equation. This is called balancing the equation. For example:
- In the reaction
there are 4 hydrogen atoms and 2 oxygen atoms on both sides, so it’s balanced.
Why are Chemical Reactions Important?
- Energy Production: Reactions like combustion (burning fuel) release energy that powers cars, factories, and homes.
- Life Processes: Many reactions happen inside our bodies (like digestion), helping us live and function.
- Everyday Products: Chemical reactions are used to make everything from soap to medicines to cleaning products.
Key Takeaways
- Atomic Structure:
- Atoms are made of protons, neutrons, and electrons.
- The atomic number tells you how many protons are in an atom, which determines the element.
- Organic Chemistry:
- The study of carbon-based compounds.
- Organic compounds are the building blocks of life and many everyday products.
- Chemical Reactions:
- Chemical reactions change substances by rearranging atoms.
- There are different types of reactions like combination, decomposition, displacement, and combustion.
Tags: acetic acid, alcohols, alkanes, alkenes, alkynes, atomic number, atomic structure, Atomic Structure: atom, balancing equations, bonds break, bonds form, carbon atom, carbon atoms, carbon dioxide, carbon-based compounds, carboxylic acids, cleaning products, combination reaction, combustion reaction, copper sulfate, decomposition reaction, digestion, displacement reaction, electrons, element, energy levels, energy production, ethanol, ethene, ethyne, fats, fuels, functional groups, hydrocarbons, hydrogen, hydrogen peroxide, life Chemical Reactions: chemical reactions, mass number, matter Organic Chemistry: organic chemistry, medicines, methane, negatively charged, neutral particles, neutrons, nitrogen, nucleus, orbit, Organic Chemistry, oxygen, plastics, positively charged, products, proteins, protons, reactants, shells, soap, sugars, water, zinc


