Elements and the Periodic Table Explained in Simple Terms
Everything around us – from the air we breathe to the food we eat – is made up of elements. But what exactly are elements, and how do they fit together? The answer is the Periodic Table, a chart that organizes all the known elements based on their properties. Let’s dive in and understand these concepts step by step
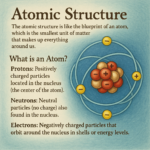
What Are Elements?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. Each element is made up of atoms, and the type of atom determines the element.
- Atoms are the basic building blocks of everything. Each atom has a nucleus (center) made of protons and neutrons, with electrons orbiting around the nucleus.
- Each element has its own type of atom. For example, an atom of carbon is different from an atom of oxygen because it has a different number of protons, neutrons, and electrons.
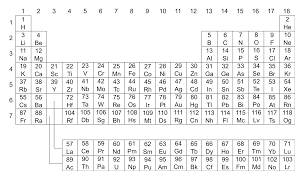
What is the Periodic Table?
The Periodic Table is a chart that organizes all known elements based on their atomic number (number of protons) and chemical properties.
- Atomic number: This is the number of protons in an atom. It determines what element the atom is. For example, hydrogen has 1 proton, so its atomic number is 1.
The Periodic Table is arranged in rows (called periods) and columns (called groups or families).
- Rows (Periods): The elements in each row have the same number of electron shells. As you move from left to right across a period, the elements change in properties.
- Columns (Groups/Families): Elements in the same column have similar chemical properties because they have the same number of electrons in their outer shell.
How is the Periodic Table Organized?
- Metals, Nonmetals, and Metalloids
- Metals: Most elements in the Periodic Table are metals. They are shiny, conduct electricity, and are usually solid at room temperature. Examples: Iron (Fe), Gold (Au).
- Nonmetals: Nonmetals are found on the right side of the Periodic Table. They are usually gases or brittle solids and don’t conduct electricity well. Examples: Oxygen (O), Nitrogen (N).
- Metalloids: Metalloids have properties of both metals and nonmetals. They are found between metals and nonmetals on the Periodic Table. Example: Silicon (Si).
- Groups (Columns)
- Group 1 (Alkali Metals): These are very reactive metals, like Lithium (Li) and Sodium (Na).
- Group 2 (Alkaline Earth Metals): These metals are also reactive but not as much as Alkali Metals. Example: Magnesium (Mg).
- Group 17 (Halogens): These are nonmetals, very reactive, and often form salts with metals. Examples: Chlorine (Cl), Fluorine (F).
- Group 18 (Noble Gases): These are very stable and do not react easily with other elements. Examples: Helium (He), Neon (Ne).
- Periods (Rows)
- As you move across a period from left to right, the elements become less metallic and more nonmetallic. The properties of the elements change gradually.
Key Parts of the Periodic Table:
- Element Symbol: Each element is represented by a one- or two-letter symbol. For example, O stands for oxygen, and H stands for hydrogen.
- Atomic Number: The number of protons in an atom of the element. For example, hydrogen’s atomic number is 1, meaning it has 1 proton in its atom.
- Atomic Mass: This is the average mass of an element’s atoms, considering the different isotopes. It is usually found beneath the element symbol.
| Atomic Number | Element Name | Symbol | |
| 1 | Hydrogen | H | |
| 2 | Helium | He | |
| 3 | Lithium | Li | |
| 4 | Beryllium | Be | |
| 5 | Boron | B | |
| 6 | Carbon | C | |
| 7 | Nitrogen | N | |
| 8 | Oxygen | O | |
| 9 | Fluorine | F | |
| 10 | Neon | Ne | |
| 11 | Sodium | Na | |
| 12 | Magnesium | Mg | |
| 13 | Aluminum | Al | |
| 14 | Silicon | Si | |
| 15 | Phosphorus | P | |
| 16 | Sulfur | S | |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og | |
How the Periodic Table Helps Us:
- Predicting Properties: By looking at where an element is on the Periodic Table, we can predict some of its properties. For example, elements in the same group often have similar reactions with other elements.
- Understanding Reactions: Knowing the elements and their properties helps scientists predict how they will react with each other. For example, when hydrogen (H) reacts with oxygen (O), it forms water (H2O).
- New Discoveries: The Periodic Table helps chemists find new elements and understand their behavior in the world.
Conclusion:
The Periodic Table is a powerful tool that helps scientists understand how elements interact and form the world around us. Each element has its own unique properties, but when you look at the table, you can see how they are connected based on their atomic number and characteristics. Whether it’s a shiny metal like Gold (Au) or a gas like Oxygen (O), elements make up everything in the universe.