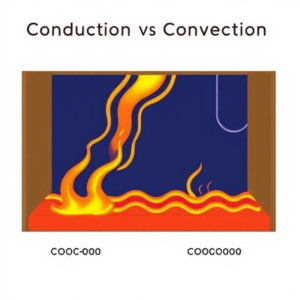
What is Thermal Conductivity?
Thermal conductivity is a property of a material that indicates how well it can conduct heat. In simple terms, it measures how easily heat passes through a material. Some materials allow heat to flow through them quickly, while others act as barriers to heat transfer.
- Good conductors of heat let heat flow through them easily (e.g., metals).
- Poor conductors (or insulators) resist the flow of heat (e.g., wood, rubber, plastics).
Key Elements to Understand Thermal Conductivity
- Heat Transfer:
Heat naturally moves from a hotter object to a cooler one until both objects reach the same temperature. Thermal conductivity measures how efficiently this heat transfer occurs within a material. - Good Conductors vs. Poor Conductors:
- Good Conductors: These materials allow heat to pass through easily. Examples include metals such as:
- Copper
- Aluminum
- Poor Conductors (Insulators): These materials do not allow heat to pass through easily. Examples include:
- Wood
- Rubber
- Plastics
- Good Conductors: These materials allow heat to pass through easily. Examples include metals such as:
Why Thermal Conductivity Matters
- High Thermal Conductivity (Good Conductors):
If you want to heat something up quickly, such as a frying pan, you’ll want a material with high thermal conductivity. Metals like copper and aluminum are excellent choices because they allow heat to spread rapidly. - Low Thermal Conductivity (Insulators):
If you want to keep something warm or insulated, like a thermos or oven mitt, you need a material with low thermal conductivity. Insulating materials like wool or Styrofoam trap heat and prevent it from escaping.
Examples of Thermal Conductivity
- Metal Spoon in a Hot Cup of Coffee:
When you place a metal spoon in a hot cup of coffee, you’ll feel the spoon becoming hot quickly. This happens because metal has high thermal conductivity, allowing heat from the coffee to transfer quickly up the spoon. - Wooden Handle of a Pan:
If you touch the wooden handle of a hot pan, it won’t feel as hot as the metal part. That’s because wood has low thermal conductivity, so it doesn’t allow heat to pass through easily.
Summary
- Thermal conductivity is a measure of how well a material can conduct or transfer heat.
- Materials like metals (e.g., copper, aluminum) are good conductors, allowing heat to pass through easily.
- Materials like wood and plastics are poor conductors (insulators), resisting heat transfer.
- Choosing the right material for thermal conductivity depends on whether you want to efficiently transfer heat or keep something hot or cold.
Understanding thermal conductivity is essential for designing systems and selecting materials for a wide range of applications, from cooking utensils to thermal insulation.